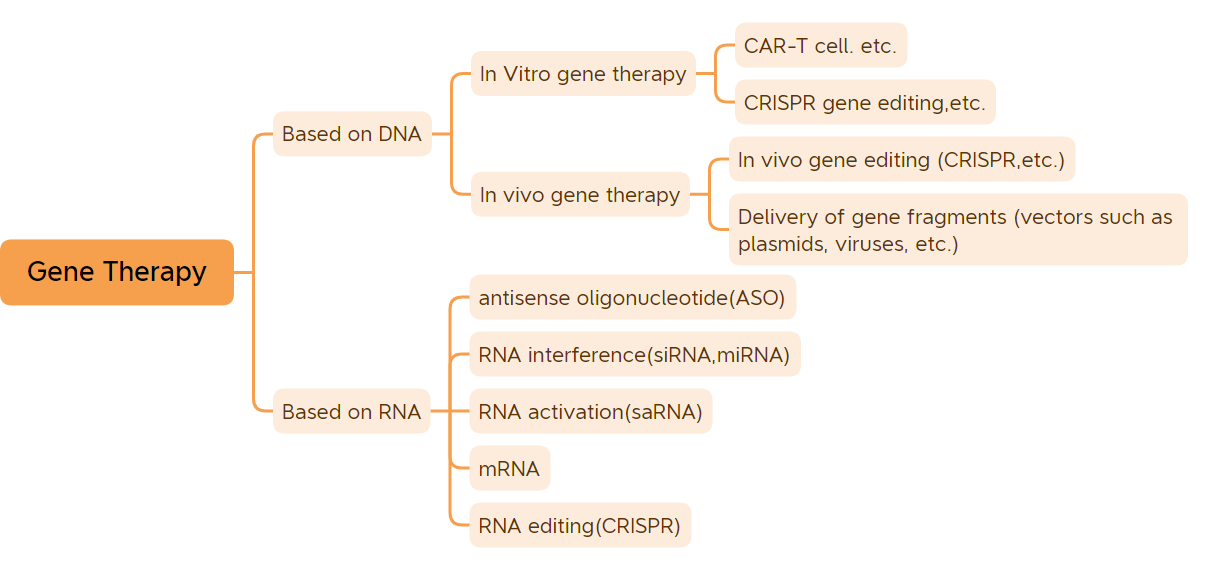
ʻO nā genes nā ʻāpana genetic kumu e hoʻomalu i nā ʻano.Ma waho aʻe o nā genes o kekahi mau maʻi maʻi, i haku ʻia me RNA, ua haku ʻia nā genes o ka hapa nui o nā meaola me ka DNA..ʻO ka hapa nui o nā maʻi o nā mea ola ma muli o ka pilina ma waena o nā genes a me ke kaiapuni.Hiki i ka Gene therapy ke ho'ōla a ho'ēmi i nā maʻi he nui.Manaʻo ʻia ʻo Gene therapy he hoʻololi i ke kahua o ka lāʻau lapaʻau a me ka lāʻau lapaʻau.ʻO nā lāʻau lapaʻau Gene therapy ma ke ʻano ākea e pili ana i nā lāʻau DNA-modified DNA (e like me nā lāʻau lapaʻau in vivo gene therapy e pili ana i nā vectors viral, in vitro gene therapy drugs, nā lāʻau plasmid hubad, etc.) a me nā lāʻau RNA (e like me nā lāʻau antisense oligonucleotide, nā lāʻau siRNA, a me ka mRNA gene therapy, etc.);ʻO nā lāʻau lapaʻau Gene therapy ka nui o nā lāʻau lapaʻau plasmid DNA, nā lāʻau lapaʻau gene e pili ana i nā vectors viral, nā lāʻau lapaʻau gene e pili ana i nā vectors bacteria, nā ʻōnaehana hoʻoponopono gene, a me nā lāʻau lapaʻau cell no ka hoʻololi ʻana i ka gene in vitro.Ma hope o nā makahiki o ka hoʻomohala ʻana, ua hoʻokō nā lāʻau lapaʻau gene therapy i nā hopena lapaʻau paipai.(ʻAʻole helu i nā lāʻau lapaʻau DNA a me nā lāʻau lapaʻau mRNA) I kēia manawa, ua ʻae ʻia nā lāʻau lapaʻau 45 gene therapy no ke kūʻai aku ma ka honua.He 9 mau lāʻau lapaʻau gene i ʻāpono ʻia no ke kūʻai aku ʻana i kēia makahiki, me 7 mau lāʻau lapaʻau gene i ʻāpono ʻia no ke kūʻai ʻana no ka manawa mua o kēia makahiki, ʻo ia hoʻi: CARVYKTI, Amvuttra, Upstaza, Roctavian, Hemgenix, Adstiladrin a me Ebvallo, (Note: Ua ʻae ʻia nā mea ʻelua ma United States i kēia makahiki. ʻO ʻAmelika Hui Pū ʻIa i ʻAukake 2022, a ua ʻae ʻia no ke kūʻai aku ʻana e ka European Union ma 2019; .) Me ka hoʻomaka ʻana o nā huahana gene therapy a me ka hoʻomohala wikiwiki ʻana o ka ʻenehana therapy gene, e hoʻomaka ana ka lāʻau lapaʻau i kahi manawa o ka ulu wikiwiki.
Ka hoʻokaʻawale ʻana o ka lāʻau lapaʻau gene (Ke kumu kiʻi: Bio-Matrix)
Hoʻopaʻa ʻia kēia ʻatikala i nā lāʻau lapaʻau gene 45 (ʻaʻole i komo i nā lāʻau lapaʻau DNA a me nā lāʻau lapaʻau mRNA) i ʻae ʻia no ke kūʻai aku.
1. In vitro gene therapy
(1) Strimvelis
Hui: Hoʻomohala ʻia e GlaxoSmithKline (GSK).
Ka manawa i ka mākeke: Ua ʻae ʻia no ke kūʻai ʻana e ka European Union i Mei 2016.
Nā hōʻailona: No ka mālama ʻana i ka maʻi immunodeficiency hui nui (SCID).
Manaʻo: ʻO ke kaʻina hana maʻamau o kēia lapaʻau, ʻo ia ka loaʻa mua ʻana o nā pūnaewele hematopoietic ponoʻī o ka mea maʻi, hoʻonui a moʻomeheu iā lākou i loko o ka vitro, a laila e hoʻohana i ka retrovirus e hoʻokomo i kahi kope gene ADA (adenosine deaminase) i loko o nā pūnaʻi hematopoietic stem, a ma hope e hoʻokomo ʻia nā pūnaʻi ʻo Hematopoietic stem i hoʻololi ʻia i loko o ke kino.Hōʻike nā hopena lapaʻau i ka 3 makahiki ola ola o nā maʻi ADA-SCID i mālama ʻia me Strimvelis he 100%.
(2) Zalmoxis
Hui: Hana ʻia e Italia MolMed Company.
Ka manawa i ka mākeke: Loaʻa i ka mana kūʻai kūʻai kūlana mai ka European Union ma 2016.
Nā hōʻailona: Hoʻohana ʻia ia no ka adjuvant therapy o ka ʻōnaehana pale o nā mea maʻi ma hope o ka hoʻololi ʻana o ka hematopoietic stem cell transplant.
Manaʻo: ʻO Zalmoxis kahi allogeneic T cell suicide gene immunotherapy i hoʻololi ʻia e nā retroviral vectors.Ke hoʻohana nei kēia ʻano i nā vectors retroviral e hoʻololi genetically i nā cell T allogeneic, no laila e hōʻike ka genetically modified T cell 1NGFR a me HSV-TK Mut2 i nā genes pepehi kanaka e hiki ai i nā kānaka ke hoʻohana i ka ganciclovir (ganciclovir) lāʻau i kēlā me kēia manawa e pepehi i nā pūnaewele T e hoʻopilikia ai i ka pale ʻana o ka GVHD, a hāʻawi i nā maʻi postoperative haploidentical reconstruction.
(3) Invossa-K
Hui: Hoʻomohala ʻia e TissueGene (KolonTissueGene).
Ka manawa i ka mākeke: Apono ʻia no ka papa inoa ʻana ma South Korea i Iulai 2017.
Nā hōʻailona: No ka mālama ʻana i ka maʻi o ka kuli degenerative.
Manaʻo: ʻO Invossa-K kahi allogeneic cell gene therapy e pili ana i nā chondrocytes kanaka.Hoʻololi ʻia nā cell allogeneic i loko o ka vitro, a hiki i nā cell i hoʻololi ʻia ke hōʻike a hūnā i ka hoʻololi ʻana i ka mea ulu β1 (TGF-β1) ma hope o ka hoʻokele intra-articular.β1), ma laila e hoʻomaikaʻi ai i nā hōʻailona o ka osteoarthritis.Hōʻike nā hopena maʻi e hiki ke hoʻomaikaʻi nui ʻo Invossa-K i ka ʻāʻī o ke kuli.Ua hoʻopau ʻia i ka makahiki 2019 e ka Korean Food and Drug Administration no ka mea ua kuhi hewa ka mea hana i nā mea i hoʻohana ʻia.
(4) Zynteglo
Hui: Ua noiʻi ʻia a hoʻomohala ʻia e ka bluebird bio.
Ka manawa i ka mākeke: Ua ʻae ʻia e ka European Union no ke kūʻai aku ʻana ma 2019, a ua ʻae ʻia e ka FDA no ke kūʻai aku ʻana ma ʻAmelika Hui Pū ʻIa i ʻAukake 2022.
Nā hōʻailona: No ka mālama ʻana i ka β-thalassemia e pili ana i ka transfusion.
Manaʻo: ʻO Zynteglo kahi lentiviral in vitro gene therapy e hoʻokomo i kahi kope hana o ka gene β-globin maʻamau (βA-T87Q-globin gene) i loko o nā pūnaewele hematopoietic i lawe ʻia mai ka mea maʻi ma o ka lentiviral vector, a laila hoʻohui hou i kēia mau genetically modified autologous hematopoietic stem cell i loko o ka mea maʻi.I ka manawa e loaʻa ai i ka mea maʻi kahi gene βA-T87Q-globin maʻamau, hiki iā lākou ke hana i ka protein HbAT87Q maʻamau, hiki ke hōʻemi a hoʻopau paha i ka pono no ka hoʻokahe koko.He lāʻau lapaʻau i hoʻokahi manawa i hoʻolālā ʻia e hoʻololi i ke kahe koko o ke ola a me nā lāʻau lapaʻau no ka poʻe maʻi 12 makahiki a ʻoi aku.
(5) Skysona
Hui: Ua noiʻi ʻia a hoʻomohala ʻia e ka bluebird bio.
ʻO ka manawa e kūʻai aku ai: Ua ʻae ʻia e ka European Union no ke kūʻai aku ʻana ma Iulai 2021, a ʻae ʻia e ka FDA no ke kūʻai aku ʻana ma United States i Kepakemapa 2022.
Nā hōʻailona: No ka mālama ʻana i ka cerebral adrenoleukodystrophy (CALD).
Manaʻo: ʻO Skysona gene therapy ʻo ia wale nō ka lāʻau lapaʻau i ʻae ʻia no ka mālama ʻana i ka cerebral adrenoleukodystrophy (CALD).ʻO Skysona (elivaldogene autotemcel, Lenti-D) he hematopoietic stem cell lentiviral in vitro gene therapy Lenti-D.Penei ke kaʻina hana maʻamau o ka lāʻau: autologous hematopoietic stem cell i waho o ka mea maʻi, transduced a hoʻololi ʻia e ka lentivirus e lawe ana i ka gene ABCD1 kanaka in vitro, a laila hoʻihoʻi hou ʻia i ka mea maʻi.Hoʻohana ʻia ia e mālama i nā maʻi ma lalo o ka makahiki 18, e lawe ana i nā hoʻololi gene ABCD1, a me CALD.
(6) Kymriah
COMPANY: Hoʻomohala ʻia e Novartis.
Ka manawa i ka mākeke: ʻApono ʻia e ka FDA no ke kūʻai aku ma ʻAukake 2017.
Nā hōʻailona: Hoʻomaʻamaʻa i ka maʻi leukemia lymphoblastic acute B-cell acute a me DLBCL i hoʻihoʻi ʻia a refractory.
Manaʻo: ʻO Kymriah kahi lāʻau lapaʻau lentiviral in vitro gene therapy, ʻo ka lāʻau lapaʻau CAR-T mua i ʻae ʻia no ka kūʻai ʻana ma ka honua, e kuhikuhi ana i ka CD19, a me ka hoʻohana ʻana i ka 4-1BB co-stimulatory factor.Ua kūʻai ʻia ma $475,000 ma US a me $313,000 ma Iapana.
(7) Yescarta
Hui: Hoʻomohala ʻia e Kite Pharma, he lālā o Gileada (GILD).
Ka manawa i ka mākeke: Apono ʻia e ka FDA no ke kūʻai ʻana ma ʻOkakopa 2017;Ua hoʻokomo ʻo Fosun Kite i ka ʻenehana Yescarta mai Kite Pharma a hana ʻia ma Kina ma hope o ka loaʻa ʻana o ka ʻae.Aponoia no ka papa inoa ma ka aina.
Nā hōʻailona: No ka mālama ʻana i ka lymphoma B-cell nui.
Manaʻo: ʻO Yescarta kahi retroviral in vitro gene therapy, ʻo ia ka lua o ka lāʻau lapaʻau CAR-T i ʻae ʻia ma ka honua.Kuhi ia i ka CD19 a hoʻohana i ka costimulator o CD28.He $373,000 ke kumu kūʻai ma ʻAmelika Hui Pū ʻIa.
(8) Tecartus
Hui: Hoʻomohala ʻia e Gileada (GILD).
Ka manawa i ka mākeke: ʻApono ʻia e ka FDA no ke kūʻai ʻana ma Iulai 2020.
Nā hōʻailona: No ka lymphoma cell mantle i hoʻihoʻi hou ʻia a i ʻole refractory.
Manaʻo: ʻO Tecartus kahi autologous cell therapy CAR-T e kuhikuhi ana i ka CD19, a ʻo ia ke kolu o ka lāʻau lapaʻau CAR-T i ʻae ʻia no ke kūʻai aku ʻana ma ka honua.
(9) Breyanzi
COMPANY: Hoʻomohala ʻia e Bristol-Myers Squibb (BMS).
Ka manawa i ka mākeke: ʻAe ʻia e ka FDA no ke kūʻai aku ma Pepeluali 2021.
Nā hōʻailona: Relapsed a refractory (R/R) nui B-cell lymphoma (LBCL).
Manaʻo: Breyanzi he in vitro gene therapy e pili ana i ka lentivirus, ka hā o ka lāʻau CAR-T i ʻae ʻia no ke kūʻai ʻana ma ka honua, e kuhikuhi ana i ka CD19.ʻO ka ʻae ʻana o Breyanzi he mea nui ia no Bristol-Myers Squibb i ke kahua o ka immunotherapy cellular, i loaʻa iā ia i ka wā i loaʻa ai iā Celgene no $ 74 biliona ma 2019.
(10) Abecma
Hui: Hoʻokumu pū ʻia e Bristol-Myers Squibb (BMS) a me bluebird bio.
Ka manawa i ka mākeke: ʻApono ʻia e ka FDA no ke kūʻai aku ma Malaki 2021.
Nā hōʻailona: hoʻihoʻi hou a i ʻole refractory multiple myeloma.
Manaʻo: ʻO Abecma kahi lāʻau lapaʻau in vitro e pili ana i ka lentivirus, ka lāʻau lapaʻau CAR-T mua o ka honua e kuhikuhi ana iā BCMA, a ʻo ka lima o ka lāʻau CAR-T i ʻae ʻia e FDA.ʻO ke kumu o ka lāʻau lapaʻau ʻo ia ka hōʻike ʻana i nā mea loaʻa chimeric BCMA ma nā keʻena T ponoʻī o ka mea maʻi ma o ka hoʻololi ʻana o ka lentivirus-mediated gene in vitro.Hoʻomaʻamaʻa e hoʻopau i nā pūnaewele T i hoʻololi ʻole ʻia i nā maʻi, a laila hoʻohui hou i nā pūnaewele T i hoʻololi ʻia, ka mea e ʻimi a pepehi i ka BCMA-expressing cancer cells i nā maʻi.
(11) Libmeldy
COMPANY: Hoʻomohala ʻia e Orchard Therapeutics.
Ka manawa i ka mākeke: ʻApono ʻia e ka European Union no ka papa inoa ma Dekemaba 2020.
Nā hōʻailona: No ka mālama ʻana i ka metachromatic leukodystrophy (MLD).
Manaʻo: ʻO Libmeldy kahi lāʻau lapaʻau e pili ana i nā cell CD34+ autologous i hoʻololi ʻia i loko o ka vitro e lentivirus.Hōʻike ka ʻikepili maʻi e hiki ke hoʻololi maikaʻi i ka infusion intravenous o Libmeldy i ke ʻano o ka MLD hoʻomaka mua i ka hoʻohālikelike ʻia me ka hōʻino kino a me ka cognitive i nā maʻi i mālama ʻole ʻia o ka makahiki like.
(12) Benoda
Hui: Hoʻomohala ʻia e WuXi Giant Nuo.
Ka manawa i ka mākeke: Ua ʻae ʻia e NMPA i Kepakemapa 2021.
Nā hōʻailona: Lapaʻau ʻana i nā poʻe maʻi makua me ka lymphoma B-cell nui (r/r LBCL) i hoʻihoʻi hou ʻia ma hope o ka laina ʻelua a i ʻole ka ʻōnaehana systemic therapy.
Manaʻo: ʻO Beinoda kahi anti-CD19 CAR-T gene therapy, a ʻo ia hoʻi ka huahana kumu o WuXi Juro Company.ʻO ia ka lua o ka huahana CAR-T i ʻāpono ʻia ma Kina, koe wale no ka relapsed/refractory nui B-cell lymphoid WuXi Giant Nuo hoʻolālā hoʻi e hoʻomohala i Regiorensai injection no ka mālama ʻana i nā hōʻailona ʻē aʻe he nui, e like me ka follicular lymphoma (FL), mantle cell lymphoma (MCL), lymphocytic leukemia (CLL) maʻi , second-line lymphoma diffuse a me BALL lymphoma nui.
(13) CARVYKTI
Hui: ʻO ka huahana mua a Legend Biotech i ʻāpono ʻia no ke kūʻai ʻana.
Ka manawa e kuai ai: Aponoia e ka FDA no ke kuai ana ma Feberuari 2022.
Nā hōʻailona: no ka mālama ʻana i ka myeloma lehulehu (R/R MM).
Manaʻo: ʻO CARVYKTI (ciltacabtagene autoleucel, Cilta-cel no ka pōkole) he CAR-T cell immune gene therapy me ʻelua mau antibodies hoʻokahi-domain e kuhikuhi ana i ka B-cell maturation antigen (BCMA).Hōʻike ka ʻikepili i ka CARVYKTI I nā poʻe maʻi me ka myeloma hou a i ʻole refractory i loaʻa i nā lāʻau lapaʻau ʻehā a ʻoi aʻe paha (me ka proteasome inhibitors, immunomodulators a me anti-CD38 monoclonal antibodies), ua hōʻike ʻia kahi helu pane holoʻokoʻa o 98%.
(14)Ebvallo
COMPANY: Hoʻomohala ʻia e Atara Biotherapeutics.
ke Komisina ʻEulopa (EC) no ka kūʻai ʻana ma Dekemaba 2022, ʻo ia ka honua T cell therapy mua i ʻae ʻia no ka kūʻai ʻana.
Nā hōʻailona: Ma ke ʻano he monotherapy no ka maʻi maʻi Epstein-Barr (EBV) e pili ana i ka maʻi lymphoproliferative post-transplantation (EBV+PTLD), pono nā poʻe maʻi e mālama ʻia he mau mākua a me nā keiki ma mua o 2 mau makahiki i loaʻa ma mua o hoʻokahi lāʻau lapaʻau ʻē aʻe.
Manaʻo: ʻO Ebvallo kahi allogeneic EBV-specific universal T-cell gene therapy e hoʻopaʻa a hoʻopau i nā cell i hoʻopili ʻia e EBV ma kahi ʻano i kaupalena ʻia e HLA.Hoʻokumu ʻia ka ʻae ʻia o kēia lāʻau lapaʻau i nā hopena o ka pivotal phase 3 clinical trial study, a ua hōʻike nā hopena i ka ORR o ka hui HCT a me ka hui SOT he 50%.He 26.3% ka huina kalahala piha (CR), he 23.7% ka hapa kala (PR), a he 1.1 mahina ka median time to remission (TTR).ʻO nā maʻi 19 i loaʻa i ke kala ʻana, ua loaʻa iā 11 ka lōʻihi o ka pane (DOR) ma mua o 6 mau mahina.Eia hou, ma ke ʻano o ka palekana, ʻaʻohe hopena ʻino e like me ka maʻi graft-versus-host (GvHD) a i ʻole Ebvallo-related cytokine release syndrome.
2. In vivo gene therapy ma muli o nā vectors viral
(1) Gendicine/Jin Sheng
Hui: Hoʻomohala ʻia e Shenzhen Saibainuo Company.
Ka manawa e kūʻai aku ai: Ua ʻae ʻia e hoʻopaʻa inoa ʻia ma Kina i ka makahiki 2003.
Nā hōʻailona: No ka mālama ʻana i ke poʻo a me ka ʻāʻī squamous cell carcinoma.
'Ōlelo Aʻo: Recombinant human p53 adenovirus injection Gendicine/Jinyousheng he adenovirus vector gene therapy drug me nā kuleana waiwai naʻauao kūʻokoʻa nona ka Shenzhen Saibainuo Company.Hoʻokumu ʻia ke kanaka ʻano 5 adenovirus me ke ʻano adenovirus kanaka 5. ʻO ka mua ke kumu nui no ka hopena anti-tumor o ka lāʻau lapaʻau, a ʻo ka mea hope ke hana nui ma ke ʻano he mea lawe.Lawe ka adenovirus vector i ka therapeutic gene p53 i loko o ka cell target, e hōʻike ana i ka tumor suppressor gene p53 i loko o ka cell target, a me kāna hōʻike gene Hiki i ka huahana ke hoʻoponopono i nā ʻano genes anti-cancer a hoʻohaʻahaʻa i nā hana o nā ʻano oncogenes, a laila e hoʻonui ai i ka hopena suppressor tumor o ke kino a hoʻokō i ke kumu o ka pepehi ʻana i nā ʻōpū.
(2) Rigvir
Hui: Kūkulu ʻia e Latima Company, Latvia.
Ka manawa hoʻopaʻa inoa: Apono ʻia no ka papa inoa ʻana ma Latvia i ka makahiki 2004.
Nā hōʻailona: No ka mālama ʻana i ka melanoma.
Manaʻo: He lāʻau lapaʻau ʻo Rigvir i hoʻokumu ʻia ma ka genetically modified enterovirus vector ECHO-7.I kēia manawa, ua lawe ʻia ka lāʻau lapaʻau ma Latvia, Estonia, Polani, Armenia, Belarus, a me nā mea ʻē aʻe, a ke hoʻopaʻa inoa ʻia nei ʻo EMA ma nā ʻāina EU.Ua hōʻike ʻia nā hihia lapaʻau i nā makahiki he ʻumi i hala aku nei he palekana a maikaʻi ʻo Rigvir oncolytic virus, a hiki ke hoʻonui i ke ola o nā maʻi melanoma e 4-6 mau manawa.Eia kekahi, pili kēia lāʻau lapaʻau i nā ʻano maʻi maʻi ʻē aʻe, e like me ka maʻi maʻi colorectal, ka maʻi pancreatic, ka maʻi maʻi maʻi maʻi maʻi maʻi maʻi maʻi maʻi maʻi maʻi maʻi, ka maʻi maʻi prostate, ka maʻi maʻi ʻāʻī, ka maʻi ʻōpū, lymphosarcoma, etc.
(3) Oncorine
Hui: Hoʻomohala ʻia e Shanghai Sanwei Biological Company.
Ka manawa i ka mākeke: Ua ʻae ʻia e hoʻopaʻa inoa ʻia ma Kina i ka makahiki 2005.
Nā hōʻailona: ka mālama ʻana i nā ʻōpū o ke poʻo a me ka ʻāʻī, ka maʻi ʻaʻai, ka maʻi maʻi pancreatic, ka maʻi maʻi ʻāʻī a me nā maʻi maʻi ʻē aʻe.
Manaʻo: ʻO Oncorine (安科瑞) kahi huahana oncolytic virus gene therapy e hoʻohana ana i ka adenovirus ma ke ʻano he mea lawe.Loaʻa ka oncolytic adenovirus, hiki ke hoʻopili kikoʻī ʻia i loko o ka p53 gene deficient a i ʻole nā maʻi maʻi ʻino, e alakaʻi ana i ka lysis o nā pūnaewele tumo, a laila e pepehi ai i nā ʻōpū tumo.me ka hoʻopōʻinoʻole i nā pūnaewele maʻamau.Ua hōʻike ʻia nā haʻawina hoʻomaʻamaʻa he palekana maikaʻi a maikaʻi ʻo Ankerui no nā ʻano maʻi maʻi ʻino.
(4) Glybera
Hui: Hoʻomohala ʻia e uniQure.
Ka manawa i ka mākeke: Apono ʻia no ka papa inoa ʻana ma ʻEulopa ma 2012.
Nā hōʻailona: Hoʻomaʻamaʻa i ka lipoprotein lipase deficiency (LPLD) me nā maʻi koʻikoʻi a i ʻole ka hoʻi hou ʻana o ka maʻi pancreatitis ʻoiai ka ʻai ʻana i ka momona.
Manaʻo: ʻO Glybera (alipogene tiparvovec) kahi lāʻau lapaʻau gene ma muli o AAV, e hoʻohana ana i ka AAV ma ke ʻano he mea lawe e hoʻohuli i ka gene therapeutic LPL i loko o nā ʻiʻo ʻiʻo, i hiki i nā cell pili ke hana i kahi lipoprotein lipase.Ua hoʻokuʻu ʻia ka lāʻau lapaʻau mai ka mākeke ma 2017. ʻO ke kumu o kona haʻalele ʻana paha e pili ana i nā kumu ʻelua: ke kumu kūʻai kiʻekiʻe a me ka noi palena palena.ʻO ke kumukūʻai maʻamau o ka lāʻau lapaʻau he kiʻekiʻe e like me US $ 1 miliona, a hoʻokahi wale nō mea maʻi i kūʻai a hoʻohana iā ia i kēia manawa.ʻOiai ua hoʻihoʻi ka hui ʻinikua olakino iā US $900,000 no ia mea, he mea kaumaha nui nō hoʻi ia no ka hui ʻinikua.Eia kekahi, ʻo nā hōʻailona i manaʻo ʻia e ka lāʻau lapaʻau he mea liʻiliʻi loa, me ka nui o ka hanana ma kahi o 1 i 1 miliona a me kahi kiʻekiʻe o ka misdiagnosis.
(5) Imlygic
Hui: Kūkulu ʻia e Amgen.
Ka manawa i ka mākeke: Ma 2015, ua ʻae ʻia e hoʻopaʻa inoa ʻia ma ʻAmelika Hui Pū ʻIa a me ʻEulopa.
Nā hōʻailona: Lapaʻau ʻana i nā ʻeha melanoma ʻaʻole hiki ke wehe loa ʻia e ka ʻoki ʻana.
Manaʻo: ʻO Imlygic kahi maʻi maʻi herpes simplex type 1 i hoʻololi ʻia e ka ʻenehana genetic (ka holoi ʻana i kāna mau ʻāpana gene ICP34.5 a me ICP47, a me ka hoʻokomo ʻana i ke kanaka granulocyte macrophage colony-stimulating factor GM-CSF gene i loko o ka maʻi) (HSV-1) ʻo ka oncolytic virus ka mua o ka FDA-approved oncolytic virus.ʻO ke ʻano o ka hoʻoponopono ʻana he intralesional injection, hiki ke hoʻopili pololei ʻia i loko o nā maʻi melanoma e hana ai i ka haki ʻana o nā cell tumo, hoʻokuʻu i nā antigens i loaʻa mai i ka tumo a me GM-CSF, a hoʻoikaika i nā pane kūʻokoʻa anti-tumor.
(6) Luxturna
Hui: Hoʻomohala ʻia e Spark Therapeutics, he lālā o Roche.
Ka manawa i ka mākeke: Ua ʻae ʻia no ke kūʻai aku ʻana e ka FDA ma 2017, a laila ʻae ʻia no ke kūʻai aku ma ʻEulopa i 2018.
Nā hōʻailona: No ka mālama ʻana i nā keiki a me nā poʻe mākua i nalowale ka ʻike ma muli o ka hoʻololi ʻana i nā gene RPE65 kope ʻelua akā mālama i ka helu kūpono o nā cell retinal ola.
'Ōlelo Aʻo: ʻO Luxturna kahi lāʻau hoʻokele gene i hoʻokumu ʻia e AAV i lawelawe ʻia e ka subretinal injection.Hoʻohana ka gene therapy i ka AAV2 ma ke ʻano he mea lawe e hoʻokomo i kahi kope hana o ka gene RPE65 maʻamau i loko o nā sela retinal o ka mea maʻi, no laila e hōʻike nā cell pili i ka protein RPE65 maʻamau, e hoʻoponopono ai i ka nele o ka protein RPE65 o ka mea maʻi, a laila e hoʻomaikaʻi ai i ka ʻike o ka mea maʻi.
(7) Zolgensma
Hui: Hoʻomohala ʻia e AveXis, kahi hui o Novartis.
Ka manawa i ka mākeke: ʻApono ʻia e ka FDA no ke kūʻai aku ma Mei 2019.
Nā hōʻailona: Hoʻomaʻamaʻa i nā maʻi maʻi Spinal Muscular Atrophy (SMA) ma lalo o 2 mau makahiki.
Manaʻo: ʻO Zolgensma kahi lāʻau lapaʻau e pili ana i ka vector AAV.ʻO kēia lāʻau lapaʻau ʻo ia wale nō ka hoʻolālā lapaʻau no ka spinal muscular atrophy i ʻae ʻia no ke kūʻai ʻana ma ka honua.ʻO ka wehe ʻana o ka lāʻau lapaʻau e wehe i kahi manawa hou i ka mālama ʻana i ka spinal muscular atrophy.ʻaoʻao, he holomua holomua.Hoʻohana kēia gene therapy i ka vector scAAV9 e hoʻokomo i ka gene SMN1 maʻamau i loko o ka mea maʻi ma o ka infusion intravenous e hana i ka protein SMN1 maʻamau, ma laila e hoʻomaikaʻi ai i ka hana o nā cell i hoʻopili ʻia e like me nā neurons motor.Ma ka hoʻohālikelike ʻana, pono nā lāʻau lapaʻau SMA Spinraza a me Evrysdi i ka dosing mau manawa lōʻihi.Hāʻawi ʻia ʻo Spinraza e ka spinal injection i kēlā me kēia ʻehā mahina, a ʻo Evrysdi kahi lāʻau lapaʻau waha i kēlā me kēia lā.
(8) Delytact
Hui: Hoʻomohala ʻia e Daiichi Sankyo Company Limited (TYO: 4568).
ʻO ka manawa e mākeke: ʻAe ʻia ke kūlana mai ke Kuhina Ola, Labour a Welfare (MHLW) o Iapana i Iune 2021.
Nā hōʻailona: No ka mālama ʻana i ka glioma malignant.
Manaʻo: ʻO Delytact ka hā o ka oncolytic virus gene therapy product i ʻāpono ʻia ma ka honua holoʻokoʻa, a ʻo ka hua mua oncolytic virus i ʻāpono ʻia no ka mālama ʻana i ka glioma malignant.ʻO Delytact kahi maʻi maʻi herpes simplex type 1 (HSV-1) i hoʻomohala ʻia e Kauka Todo a me nā hoa hana.Hoʻokomo ʻo Delytact i nā hoʻololi hoʻopau hou i loko o ka genome G207 o ka lua o ka hanauna HSV-1, e hoʻonui ana i kāna replication koho i loko o nā maʻi maʻi maʻi a me ka hoʻokomo ʻana i nā pane pale anti-tumor ʻoiai e mālama ana i ka palekana kiʻekiʻe.ʻO Delytact ke kolu o ka hanauna mua oncolytic HSV-1 e hele nei i ka loiloi lapaʻau.Hoʻokumu ʻia ka ʻae ʻana o Delytact ma Iapana ma kahi hoʻokolohua lapaʻau hoʻokahi lima lima.I nā poʻe maʻi me ka glioblastoma hou, ua hoʻokō ʻo Delytact i ka hopena mua o ka helu ola hoʻokahi makahiki, a ua hōʻike nā hopena ua hōʻike ʻo Delytact i ka maikaʻi ʻoi aku ka maikaʻi ma mua o G207.Ka ikaika replicative a me ka hana antitumor kiʻekiʻe.He mea maikaʻi kēia i nā ʻano maʻi maʻi maʻi o ka umauma, prostate, schwannomas, nasopharyngeal, hepatocellular, colorectal, malignant peripheral nerve sheath tumors, a me ka maʻi maʻi thyroid.
(9) Upstaza
COMPANY: Hoʻomohala ʻia e PTC Therapeutics, Inc. (NASDAQ: PTCT).
Ka manawa i ka mākeke: Apono ʻia e ka European Union no ke kūʻai aku ʻana ma Iulai 2022.
Nā hōʻailona: No ka hemahema L-amino acid decarboxylase (AADC), ua ʻae ʻia no ka mālama ʻana i nā maʻi ma mua o 18 mau mahina a ʻoi.
Manaʻo: ʻO Upstaza™ (eladocagene exuparvovec) he lāʻau lapaʻau in vivo gene me ka adeno-associated virus type 2 (AAV2) ma ke ʻano he mea lawe.Loaʻa nā maʻi i ka maʻi ma muli o ka hoʻololi ʻana i ka gene e hoʻopili ana i ka enzyme AADC.Lawe ʻo AAV2 i kahi gene olakino e hoʻopili ana i ka enzyme AADC.Loaʻa ke ʻano o ka uku gene i kahi hopena therapeutic.Ma ke kumumanaʻo, hoʻokahi hoʻokele pono no ka manawa lōʻihi.ʻO ia ka lāʻau lapaʻau mua i kūʻai ʻia i loko o ka lolo.Pili ka ʻae kūʻai aku i nā mokuʻāina lālā 27 EU, a me Iceland, Norewai a me Liechtenstein.
(10) Roctavian
Hui: Hoʻomohala ʻia e BioMarin Pharmaceutical (BioMarin).
Ka manawa i ka mākeke: ʻApono ʻia no ke kūʻai ʻana e ka European Union i ʻAukake 2022;ka mana kūʻai aku e ka UK Medicines and Healthcare products Administration (MHRA) i Nowemapa 2022.
Nā hōʻailona: No ka mālama ʻana i nā poʻe maʻi makua me ka hemophilia koʻikoʻi A ʻaʻohe moʻolelo o ka FVIII factor inhibition a maikaʻi ʻole no nā antibodies AAV5.
Manaʻo: Hoʻohana ʻo Roctavian (valoctocogene roxaparvovec) i ka AAV5 ma ke ʻano he vector a hoʻohana i ka HLP mea hoʻolaha kikoʻī o ke akepaʻa kanaka e hoʻokele i ka hōʻike o ke kanaka coagulation factor VIII (FVIII) me ka waihona B i holoi ʻia.ʻO ka hoʻoholo a ke Komisina ʻEulopa e ʻae i ka kūʻai ʻana o ka valoctocogene roxaparvovec e pili ana i ka ʻikepili holoʻokoʻa o ka papahana hoʻomohala lāʻau lapaʻau.Ma waena o lākou, ua hōʻike ʻia nā hopena o ka pae III hoʻokolohua GENER8-1 i hoʻohālikelike ʻia me ka ʻikepili o ka makahiki ma mua o ke kau inoa ʻana, ma hope o ka infusion hoʻokahi o ka valoctocogene roxaparvovec, ua hoʻemi nui ʻia ke kumu o ke kahe koko makahiki (ABR), ʻo ke alapine o ka hoʻohana ʻana i ka recombinant coagulation factor VIII (F8) ua hoʻemi ʻia ka hoʻomākaukau ʻana o ka protein i ke koko.Ma hope o 4 pule o ka lapaʻau ʻana, ua hoʻemi ʻia ka helu hoʻohana F8 makahiki o ke kumuhana a me ka ABR e koi ana i ka mālama ʻana e 99% a me 84%, kēlā me kēia, a ʻo ka ʻokoʻa ka nui o ka helu (p<0.001).Maikaʻi ka ʻike palekana, a ʻaʻohe kumuhana i ʻike i ka F8 factor inhibition, malignancy a thrombosis side effects, a ʻaʻole i hōʻike ʻia nā hopena koʻikoʻi pili i ka mālama ʻana (SAEs).
(11) Hemgenix
Hui: Hoʻomohala ʻia e UniQure Corporation.
Ka manawa i ka mākeke: ʻApono ʻia e ka FDA no ke kūʻai aku ma Nowemapa 2022.
Nā hōʻailona: No ka mālama ʻana i nā poʻe maʻi me ka hemophilia B.
Manaʻo: Hemgenix kahi lāʻau lapaʻau e pili ana i ka vector AAV5.Hoʻolako ʻia ka lāʻau lapaʻau me ka coagulation factor IX (FIX) gene variant FIX-Padua, ka mea i lawelawe ʻia me ka intravenously.Ma hope o ka hoʻoponopono ʻana, hiki i ka gene ke hōʻike i ka FIX coagulation factor i loko o ke ake a huna Ma hope o ke komo ʻana i ke koko e hoʻokō i ka hana coagulation, i hiki ai ke hoʻokō i ke kumu o ka mālama ʻana, theoretically, hoʻokahi hoʻokele pono no ka manawa lōʻihi.
(12) Adstiladrin
Hui: Hoʻomohala ʻia e Ferring Pharmaceuticals.
Ka manawa e kuai ai: Aponoia e ka FDA no ke kuai ana ma Kekemapa 2022.
Nā hōʻailona: No ka mālama ʻana i ka maʻi maʻi maʻi maʻi maʻi non-muscle-invasive (NMIBC) i pane ʻole iā Bacillus Calmette-Guerin (BCG).
Manaʻo: Adstiladrin mea he gene therapy ma muli o ka non-replicating adenoviral vector, hiki ke overexpress interferon alfa-2b protein i target cell, a lawelawe ia ma o ka urinary catheter i loko o ka ohe (hoʻokō 'ia hoʻokahi manawa i kela ekolu mahina), ka mea maʻi vector hiki pono ke hoʻopili i loko o nā keena o ka pā o ka pūpū, a laila e hoʻonui i ka hopena inter.No laila, hoʻololi kēia ʻano hana hoʻomaʻamaʻa gene no ka mea maʻi ponoʻī i loko o kahi "hale hana" liʻiliʻi e hana ana i ka interferon, a laila e hoʻonui ai i ka hiki o ka mea maʻi ke hakakā i ka maʻi kanesa.
Ua loiloi ʻia ka palekana a me ka maikaʻi o Adstiladrin i kahi noiʻi lapaʻau multicenter me nā maʻi 157 me ka NMIBC-nonresponsive BCG kiʻekiʻe.Loaʻa i nā poʻe maʻi ka Adstiladrin i kēlā me kēia ʻekolu mahina a hiki i 12 mahina, a i ʻole a hiki i ka hiki ʻole ke ʻae ʻia i ka lāʻau lapaʻau a i ʻole ka hoʻi hou ʻana o NMIBC kiʻekiʻe.ʻO ka holoʻokoʻa, 51 pakeneka o nā maʻi i kākau inoa ʻia me Adstiladrin i loaʻa i kahi pane piha (nalo o nā hōʻailona āpau o ka maʻi kanesa i ʻike ʻia ma ka cystoscopy, biopsy tissue, a me ka mimi).
3. Nā lāʻau liʻiliʻi nucleic acid
(1) Vitravene
Hui: Hoʻokumu pū ʻia e Ionis Pharma (ʻo Isis Pharma ma mua) a me Novartis.
Ka manawa i ka mākeke: Ma 1998 a me 1999, ua ʻae ʻia ia no ke kūʻai ʻana e FDA a me EU EMA.
Nā hōʻailona: No ka mālama ʻana i ka cytomegalovirus retinitis i nā maʻi maʻi HIV.
Manaʻo: ʻO Vitravene kahi lāʻau antisense oligonucleotide, ʻo ia ka lāʻau oligonucleotide mua i ʻae ʻia no ke kūʻai aku ma ka honua.I ka manawa mua o ka papa inoa, ua wikiwiki loa ka noi mākeke no nā lāʻau anti-CMV;ma hope mai, ma muli o ka hoʻomohala ʻana o ka lāʻau antiretroviral ikaika loa, ua hāʻule nui ka nui o nā hihia CMV.Ma muli o ka lohi o ka mākeke noi, ua hoʻokuʻu ʻia ka lāʻau lapaʻau ma 2002 a me 2006 Withdrawal i nā ʻāina EU a me ʻAmelika Hui Pū ʻIa.
(2) Macugen
Hui: Hoʻokumu pū ʻia e Pfizer a me Eyetech.
Ka manawa i ka mākeke: Apono ʻia no ka papa inoa ʻana ma United States i ka makahiki 2004.
Nā hōʻailona: No ka mālama ʻana i ka macular degeneration pili i ka makahiki neovascular.
Manaʻo: ʻO Macugen kahi lāʻau oligonucleotide i hoʻololi ʻia e pegylated, hiki ke hoʻopaʻa a hoʻopaʻa i ka mea ulu endothelial vascular (VEGF165 subtype), a ʻo ke ʻano hoʻokele intravitreal injection.
(3) Defitelio
Hui: Hoʻomohala ʻia e Jazz Pharmaceuticals.
Ka manawa i ka mākeke: Ua ʻae ʻia no ke kūʻai aku ʻana e ka European Union ma 2013 a ua ʻae ʻia e ka FDA no ke kūʻai aku ma Malaki 2016.
Nā hōʻailona: No ka mālama ʻana i ka maʻi hepatic veno-occlusive e pili ana me ka hana ʻole o ka renal a i ʻole ka pulmonary ma hope o ka hemopoietic stem cell transplantation.
Manaʻo: ʻO Defitelio kahi lāʻau oligonucleotide, kahi hui o nā oligonucleotides me nā waiwai plasmin.Hoʻihoʻi ʻia mai ka mākeke ma 2009 no nā kumu kalepa.
(4) Kynamro
Hui: Hoʻokumu pū ʻia e Ionis Pharma a me Kastle.
Ka manawa e kūʻai aku ai: Ma 2013, ua ʻae ʻia no ke kūʻai aku ʻana ma ʻAmelika Hui Pū ʻIa ma ke ʻano he lāʻau makua ʻole.
Nā hōʻailona: No ka mālama ʻana i ka hypercholesterolemia ʻohana homozygous.
Manaʻo: ʻO Kynamro kahi lāʻau antisense oligonucleotide, ʻo ia ka antisense oligonucleotide e kuhikuhi ana i ke kanaka apo B-100 mRNA.Hāʻawi ʻia ʻo Kynamro e like me 200 mg subcutaneously i hoʻokahi manawa i ka pule.
(5) Spinraza
Hui: Hoʻomohala ʻia e Ionis Pharmaceuticals.
Ka manawa e kuai ai: Aponoia e ka FDA no ke kuai ana ma Kekemapa 2016.
Nā hōʻailona: No ka mālama ʻana i ka spinal muscular atrophy (SMA).
Manaʻo: ʻO Spinraza (nusinersen) kahi lāʻau antisense oligonucleotide.Ma ka hoʻopaʻa ʻana i ka pūnaewele cleavage o SMN2 exon 7, hiki iā Spinraza ke hoʻololi i ka cleavage RNA o SMN2 gene, ma laila e hoʻonui ai i ka hana o ka protein SMN holoʻokoʻa.I ʻAukake 2016, ua hoʻohana ʻo BIOGEN i kāna koho e kiʻi i nā kuleana honua iā Spinraza.Ua hoʻomaka wale ʻo Spinraza i kāna hoʻokolohua lapaʻau mua i nā kānaka ma 2011. I nā makahiki 5 wale nō, ua ʻae ʻia e ka FDA no ke kūʻai aku ma 2016, e hōʻike ana i ka ʻike piha ʻana o ka FDA i kona pono.Ua ʻae ʻia ka lāʻau lapaʻau no ke kūʻai ʻana ma Kina i ʻApelila 2019. ʻO ka pōʻai holoʻokoʻa holoʻokoʻa no Spinraza ma Kina ʻoi aku ka liʻiliʻi ma mua o 6 mau mahina, a ʻo 2 mau makahiki a me 2 mau mahina mai ka manawa i ʻae ʻia ai ʻo Spinraza ma United States.ʻO ka wikiwiki o ka papa inoa ma Kina ua wikiwiki loa.No ka mea, ua hoʻopuka ka Center for Drug Evaluation i ka "Notice on Publishing the List of the First Batch of Overseas New Drugs Urgently Needed in Clinical Practice" ma Nowemapa 1, 2018, a ua hoʻokomo ʻia i loko o ka pūʻulu mua o 40 mau lāʻau lapaʻau hou no ka loiloi wikiwiki ʻana, ma waena o Spinraza i koho ʻia.
(6) Exondys 51
Hui: Hoʻomohala ʻia e AVI BioPharma (i kapa hou ʻia ʻo Sarepta Therapeutics).
Ka manawa e kūʻai aku ai: I Kepakemapa 2016, ua ʻae ʻia ia no ke kūʻai ʻana e ka FDA.
Nā hōʻailona: No ka mālama ʻana i ka Duchenne muscular dystrophy (DMD) me ka exon 51 skipping gene mutation i ka DMD gene.
Manaʻo: ʻO Exondys 51 kahi lāʻau antisense oligonucleotide, hiki i ka antisense oligonucleotide ke hoʻopaʻa i ke kūlana o exon 51 o pre-mRNA o DMD gene, ka hopena i ka hoʻokumu ʻana o ka mRNA makua, kahi ʻāpana o ka exon 51 ua kāohi ʻia ʻo Excision, no laila e hoʻoponopono i kahi ʻāpana i ka mRNA heluhelu frame, e kōkua ana i kekahi mau maʻi e hoʻomaʻamaʻa i ka protein ma mua o ka systroph maʻamau. nā hōʻailona o ka mea maʻi.
(7) Tegsedi
Hui: Hoʻomohala ʻia e Ionis Pharmaceuticals.
Ka manawa i ka mākeke: Ua ʻae ʻia no ke kūʻai ʻana e ka European Union i Iulai 2018.
Nā hōʻailona: No ka mālama ʻana i ka transthyretin amyloidosis (hATTR).
Manaʻo: ʻO Tegsedi kahi lāʻau antisense oligonucleotide e huli ana i ka transthyretin mRNA.ʻO ia ka lāʻau lapaʻau mua i ʻae ʻia ma ka honua no ka mālama ʻana i ka hATTR.Hāʻawi ʻia ia e ka subcutaneous injection.Hoʻemi ka lāʻau lapaʻau i ka hana ʻana o ka protein ATTR ma o ka huli ʻana i ka mRNA o transthyretin (ATTR), a loaʻa kahi ratio pōmaikaʻi maikaʻi i ka mālama ʻana i ka ATTR, a ua hoʻomaikaʻi nui ʻia ka neuropathy a me ka maikaʻi o ke ola o ka mea maʻi, a ua kūpono ia me nā ʻano mutation TTR, ʻAʻole i pili ka maʻi a me ka hele ʻana o ka cardiomyopathy.
(8) Onpattro
Hui: Hoʻomohala hui ʻia e Alnylam Corporation a me Sanofi Corporation.
Ka manawa i ka mākeke: ʻApono ʻia no ka papa inoa ʻana ma United States i 2018.
Nā hōʻailona: No ka mālama ʻana i ka transthyretin amyloidosis (hATTR).
Manaʻo: ʻO Onpattro kahi lāʻau lapaʻau siRNA e huli ana i ka transthyretin mRNA, e hōʻemi ana i ka hana ʻana o ka protein ATTR i loko o ke ake a hōʻemi i ka hōʻiliʻili ʻana o nā waihona amyloid i nā aʻalolo peripheral ma ka huli ʻana i ka mRNA o transthyretin (ATTR), ma laila e hoʻomaikaʻi ai a hoʻemi i nā hōʻailona maʻi.
(9) Givlaari
Hui: Hoʻomohala ʻia e Alnylam Corporation.
Ka manawa i ka mākeke: ʻApono ʻia e ka FDA no ke kūʻai ʻana i Nowemapa 2019.
Nā hōʻailona: No ka mālama ʻana i ka porphyria hepatic acute (AHP) i nā pākeke.
Manaʻo: ʻO Givlaari kahi lāʻau siRNA, ʻo ia ka lua o ka lāʻau siRNA i ʻae ʻia no ke kūʻai ʻana ma hope o Onpattro.ʻO ke ʻano o ka hoʻokele ʻana he injection subcutaneous.Hoʻopili ka lāʻau lapaʻau i ka mRNA o ka protein ALAS1, a me ka mālama ʻana i kēlā me kēia mahina me Givlaari hiki ke hoʻohaʻahaʻa i ke kiʻekiʻe o ka ALAS1 i loko o ke akepaʻa, a laila e hōʻemi i nā pae o ka neurotoxic ALA a me PBG i ka pae maʻamau, a laila e hoʻohaʻahaʻa i nā hōʻailona o ka maʻi o ka mea maʻi.Ua hōʻike ʻia nā ʻikepili i nā mea maʻi i mālama ʻia me Givlaari he 74% ka emi ʻana o ka nui o ka hopu ʻana i hoʻohālikelike ʻia i ka hui placebo.
(10) Vyondys53
COMPANY: Hoʻomohala ʻia e Sarepta Therapeutics.
Ka manawa i ka mākeke: Apono ʻia e ka FDA no ke kūʻai aku ʻana ma Dekemaba 2019.
Nā hōʻailona: No ka mālama ʻana i nā maʻi DMD me ka dystrophin gene exon 53 splicing mutation.
Manaʻo: ʻO Vyondys 53 kahi lāʻau antisense oligonucleotide, e kuhikuhi ana i ke kaʻina splicing o dystrophin pre-mRNA.Ua ʻoki hapa ʻia ʻo Exon 53, ʻo ia hoʻi, ʻaʻole i loaʻa ma ka mRNA makua, a ua hoʻolālā ʻia e hana i kahi dystrophin i ʻoki ʻia akā e hana mau ana, a laila e hoʻomaikaʻi ai i ka hiki ke hoʻoikaika kino i nā maʻi.
(11) Waylivra
Hui: Hoʻomohala ʻia e Ionis Pharmaceuticals a me kāna ʻoihana ʻo Akcea Therapeutics.
Ka manawa i ka mākeke: Ua ʻae ʻia no ke kūʻai ʻana e ka European Medicines Agency (EMA) i Mei 2019.
Nā hōʻailona: Ma ke ʻano he lāʻau adjuvant me ka mālama ʻana i ka meaʻai i nā poʻe maʻi makua me ka familial chylomicronemia syndrome (FCS).
Manaʻo: ʻO Waylivra kahi lāʻau antisense oligonucleotide, ʻo ia ka lāʻau lapaʻau mua i ʻae ʻia no ke kūʻai aku ma ka honua no ka mālama ʻana i ka FCS.
(12) Leqvio
Hui: Kūkulu ʻia e Novartis.
Ka manawa i ka mākeke: Ua ʻae ʻia e ka European Union no ke kūʻai aku ma Dekemaba 2020.
Nā hōʻailona: No ka mālama ʻana i nā pākeke me ka hypercholesterolemia mua (ʻohana heterozygous a me non-familial) a i ʻole dyslipidemia hui ʻia.
Manaʻo: ʻO Leqvio kahi lāʻau lapaʻau siRNA e kuhikuhi ana i ka PCSK9 mRNA.ʻO ia ka lāʻau siRNA mua loa o ka honua no ka hoʻohaʻahaʻa ʻana i ka cholesterol (LDL-C).Hāʻawi ʻia ia e ka subcutaneous injection.Hoʻemi ka lāʻau lapaʻau i ke kiʻekiʻe o ka protein PCSK9 ma o ka interference RNA, a laila e hōʻemi i ka pae o LDL-C.Hōʻike ka ʻikepili lapaʻau no nā poʻe maʻi ʻaʻole hiki ke hōʻemi i nā pae LDL-C i ka pae i manaʻo ʻia ma hope o ka mālama ʻana me ka nui o nā statins ʻae ʻia, hiki iā Leqvio ke hoʻemi i ka LDL-C ma kahi o 50%.
(13) ʻOxlumo
Hui: Hoʻomohala ʻia e Alnylam Pharmaceuticals.
Ka manawa i ka mākeke: Ua ʻae ʻia e ka European Union no ke kūʻai aku ma Nowemapa 2020.
Nā hōʻailona: No ka mālama ʻana i ka hyperoxaluria type 1 (PH1).
Manaʻo: ʻO Oxlumo kahi lāʻau lapaʻau siRNA e huli ana i ka hydroxyacid oxidase 1 (HAO1) mRNA, a ʻo ke ʻano o ka hoʻokele ʻana ʻo ia ka subcutaneous injection.Ua hoʻomohala ʻia ka lāʻau lapaʻau me ka hoʻohana ʻana i ka kemika stabilization hou loa o Alnylam, ʻenehana conjugation ESC-GalNAc, e hiki ai i ka subcutaneously lawelawe ʻo siRNA me ka hoʻomau a me ka ikaika.ʻO ka lāʻau lapaʻau e hoʻohaʻahaʻa aiʻole e kāohi i ka hydroxyacid oxidase 1 (HAO1) mRNA, e ho'ēmi i ke kiʻekiʻe o ka glycolate oxidase i loko o ke akepaʻa, a laila e hoʻopau i ka substrate e pono ai no ka hanaʻana i ka oxalate, e ho'ēmi i ka hana oxalate e hoʻomalu i ka holomua o ka maʻi i nā maʻi a hoʻonui i nā hōʻailona maʻi.
(14) Viltepso
Hui: Hoʻomohala ʻia e NS Pharma, kahi hui o Nippon Shinyaku.
Ka manawa i ka mākeke: ʻApono ʻia e ka FDA no ke kūʻai aku ma ʻAukake 2020.
Nā hōʻailona: No ka mālama ʻana i ka Duchenne muscular dystrophy (DMD) me ka exon 53 skipping gene mutation ma ka DMD gene.
Manaʻo: ʻO Viltepso kahi lāʻau antisense oligonucleotide e hiki ke hoʻopaʻa i ke kūlana o exon 53 o ka pre-mRNA o ka DMD gene, e hoʻoneʻe ʻia kahi hapa o exon 53 ma hope o ka hoʻokumu ʻia ʻana o ka mRNA makua, a laila e hoʻoponopono i ka ʻāpana heluhelu mRNA.
(15) Amondys 45
Hui: Hoʻomohala ʻia e Sarepta Therapeutics.
Ka manawa i ka mākeke: ʻAe ʻia e ka FDA no ke kūʻai aku ma Pepeluali 2021.
Nā hōʻailona: No ka mālama ʻana i ka Duchenne muscular dystrophy (DMD) me ka exon 45 skipping gene mutation i ka DMD gene.
Manaʻo: ʻO Amondys 45 kahi lāʻau antisense oligonucleotide, hiki i ka antisense oligonucleotide ke hoʻopaʻa i ke kūlana o exon 45 o ka pre-mRNA o DMD gene, ka hopena o ka ʻāpana o ka exon 45 i kāohi ʻia ma hope o ka hoʻokumu ʻia ʻana o ka mRNA Excision, a laila hoʻoponopono ʻāpana i ka mRNA heluhelu ʻana, e kōkua ana i nā mea maʻi e hoʻomaʻamaʻa i ka ʻōnaehana heluhelu mRNA. i nā hōʻailona o ka mea maʻi.
(16) Amvuttra (vutrisiran)
Hui: Hoʻomohala ʻia e Alnylam Pharmaceuticals.
Ka manawa e kuai ai: Aponoia e ka FDA no ke kuai ana ma Iune 2022.
Nā hōʻailona: No ka mālama ʻana i ka transthyretin amyloidosis me ka polyneuropathy (hATTR-PN) i nā pākeke.
Manaʻo: ʻO Amvuttra (Vutrisiran) kahi lāʻau lapaʻau siRNA e huli ana i ka transthyretin (ATTR) mRNA, i lawelawe ʻia e ka subcutaneous injection.Hoʻokumu ʻia ʻo Vutrisiran ma ka Alnylam's Enhanced Stability Chemistry (ESC)-GalNAc conjugate delivery platform design me ka hoʻonui ʻana i ka mana a me ka paʻa metabolic.Hoʻokumu ʻia ka ʻae ʻana o ka lāʻau lapaʻau ma ka ʻikepili 9-mahina o kāna haʻawina hauʻoli Phase III (HELIOS-A), a ʻo nā hopena holoʻokoʻa e hōʻike ana ua hoʻomaikaʻi ka lāʻau i nā hōʻailona o ka hATTR-PN, a ʻoi aku ma mua o 50% o ke kūlana o nā maʻi i hoʻohuli ʻia a hoʻōki ʻia mai ka piʻi ʻana.
4. Nā lāʻau lapaʻau gene therapy ʻē aʻe
(1) Rexin-G
Hui: Hoʻomohala ʻia e Epeius Biotech.
Ka manawa e kuai ai: I ka makahiki 2005, ua aponoia no ke kuai ana e ka Philippine Food and Drug Administration (BFAD).
Nā hōʻailona: No ka mālama ʻana i nā maʻi maʻi maʻi kūʻē i ka chemotherapy.
Manaʻo: ʻO Rexin-G kahi mea i hoʻopaʻa ʻia i ka nanoparticle injection.Hoʻokomo ʻo ia i ka cyclin G1 mutant gene i loko o nā keʻena i hoʻopaʻa ʻia ma o ka retroviral vector e pepehi pono i nā ʻōpū paʻa.ʻO ke ʻano o ka lawelawe ʻana he intravenous infusion.Ma ke ʻano he lāʻau lapaʻau i hoʻopaʻa ʻia i ka maʻi maʻi e ʻimi ikaika ana a luku i nā cell cancer metastatic, loaʻa iā ia kekahi hopena curative i nā poʻe maʻi i hāʻule ʻole i nā lāʻau maʻi maʻi ʻē aʻe, e komo pū me nā biologics.
(2) Neovasculgen
Hui: Hoʻomohala ʻia e ka Human stem cell institute.
Wā papa inoa: Ua ʻae ʻia no ka hoʻopaʻa inoa ʻana ma Rūsia ma Dekemaba 7, 2011, a laila hoʻokuʻu ʻia ma Ukraine i 2013.
Nā ʻōlelo hōʻike: No ka mālama ʻana i nā maʻi ʻāʻī o nā ʻāpana āpau, me ka ischemia o nā lālā koʻikoʻi.
Manaʻo: ʻO Neovasculgen kahi lāʻau lapaʻau e pili ana i nā plasmids DNA.Kūkulu ʻia ka gene vascular endothelial growth factor (VEGF) 165 ma ka iwi kuamoʻo plasmid a hoʻokomo ʻia i loko o nā maʻi.
(3) Collategene
Hui: Hoʻomohala hui ʻia e ke Kulanui ʻo Osaka a me nā ʻoihana ʻoihana ʻoihana.
ʻO ka manawa e kūʻai aku ai: Ua ʻae ʻia e ke Kuhina Ola, Limahana a me ka Welfare o Iapana i ʻAukake 2019.
Nā hōʻailona: Hoʻomaʻamaʻa ʻana i ka ischemia koʻikoʻi o ka ʻaoʻao haʻahaʻa.
Manaʻo: ʻO Collategene kahi lāʻau lapaʻau i hoʻokumu ʻia i ka plasmid, ʻo ka lāʻau lapaʻau home gene therapy mua i hana ʻia e AnGes, kahi hui lāʻau lapaʻau ma Iapana.ʻO ka mea nui o kēia lāʻau lapaʻau he plasmid olohelohe i loaʻa i ke kaʻina gene hepatocyte growth factor (HGF).Inā hoʻokomo ʻia ka lāʻau lapaʻau i loko o nā ʻiʻo o nā lālā haʻahaʻa, ʻo ka HGF i hōʻike ʻia e hāpai i ke kūkulu ʻana i nā kīʻaha koko hou a puni nā kīʻaha koko i pani ʻia.Ua hōʻoia nā hoʻokolohua lapaʻau i kona hopena i ka hoʻomaikaʻi ʻana i nā ʻeha.
Pehea e hiki ai iā Foregene ke kōkua i ka hoʻomohala ʻana o ka gene therapy?
Kōkua mākou i ka mālama ʻana i ka manawa screening i ka nānā ʻana nui, i ka wā mua o ka hoʻomohala ʻana i ka lāʻau siRNA.
E kipa aku i nā kikoʻī hou aku:
https://www.foreivd.com/cell-direct-rt-qpcr-kit-direct-rt-qpcr-series/
Ka manawa hoʻouna: Dec-27-2022